Materials Experts

Differential Scanning Calorimetry
(DSC 6000 – Perkin Elmer)
The differential scanning calorimeter (DSC) is a fundamental tool in thermal analysis. The information these instruments generate is used to understand amorphous and crystalline behavior, polymorph and eutectic transitions, curing and degree of cure, and many other material properties used to design, manufacture, and test products. It can be used in many industries – from pharmaceuticals and polymers, to nanomaterials and agrifood products.
DSC analysis is used to measure melting temperature, heat of fusion, latent heat of melting, reaction energy and temperature, glass transition temperature, crystalline phase transition temperature and energy, precipitation energy and temperature, denaturation temperatures, oxidation induction times, and specific heat or heat capacity. By measuring the amount of energy absorbed or released by non-corrosive sample when it is heated or cooled, DSC analysis provide quantitative and qualitative data on endothermic (heat absorption) and exothermic (heat evolution) processes.
Thermogravimetric analysis or thermal gravimetric analysis
(TGA 8000– Perkin Elmer)
Thermogravimetry Analysis (TGA) is the technology for measuring the relationship between mass and temperature (or time), under heating or cooling process, or under constant temperature, while the temperature is under control. TGA measures weight/mass change (loss or gain) and the rate of weight change as a function of temperature, time and atmosphere.
Measurements are used primarily to determine the composition of materials and to predict their thermal stability. The technique can characterize materials that exhibit weight loss or gain due to sorption/desorption of volatiles, decomposition, oxidation and reduction.
Mechanisms of Weight Change in TGA
1 Weight Loss:
-
Decomposition: The breaking apart of chemical bonds.
-
Evaporation: The loss of volatiles with elevated temperature.
-
Reduction: Interaction of sample to a reducing atmosphere (hydrogen, ammonia, etc.).
-
Desorption.
2 Weight Gain:
-
Oxidation: Interaction of the sample with an oxidizing atmosphere.
-
Absorption.
-
All of these are kinetic processes (i.e. there is a rate at which they occur).

Example:
Characterization of Polymers Using TGA W.J. Sichina, Marketing Manager – Washinton dep.edu • Compositional analysis of multi-component materials or blends • Thermal stabilities • Oxidative stabilities • Estimation of product lifetimes • Decomposition kinetics • Effects of reactive atmospheres on materials • Filler content of materials • Moisture and volatiles content
Other currents applications:
Ceramics, Metal Materials, Flame Retardant, Carbon capture, Chemical loop, Vegetable Oil Oxidative Stability, Polymer Applications, and others

Spectrum obtained can be analysis compare
We can analyze following type of samples
-
Soft polymers, Rubbers, thin film*
-
Soft powders, Pastes, Gels, Aqueous solutions
-
Surface coatings
-
Organic products
-
Proteins and other biochemical
-
Comparison of plant extract.
-
Impurity included a pure reference sample.
Fourier transform infrared
spectroscopy (FTIR )
FTIR is characterization technique used in qualitative material analysis within the biological, biomedical and chemical industry; this technique takes advantage of the vibrational transitions of a molecule and is widely used in organic synthesis, polymer science, petrochemical engineering, pharmaceutical industry and food analysis. FTIR is a method of measuring infrared absorption and emission spectra.
In organic or inorganic compounds, IR Spectroscopy is used for checking presence of functional groups. The fundamental measurement obtained in infrared spectroscopy is an infrared spectrum, which is a plot of measured infrared intensity versus wavelength (or frequency) of light. Units of cm-1 is commonly used for wavenumber.
*By transmission, analyzing of Thin Films* should be in the freestanding condition and thickness should be ≤ 50μm. Thicker film can be analyzed By ATR
Attenuated total reflection
(ATR) spectroscopy
ATR is one accessory of FTIR spectrophotometer to measure surface properties of solid or thin film samples rather than their bulk properties. Generally, ATR has a penetration depth of around 1 or 2 micrometers depending on your sample conditions.
The infrared radiation interacts with the sample through series of standing waves called evanescent waves, which penetrate the sample at each reflection point.
An evanescent wave is produced each time the infrared beam is reflected from the inside surface of the crystal. Some of the energy of evanescent wave is absorbed by the sample and reflected radiation is returned to the detector.

Fourier transform infrared
spectroscopy (FTIR )
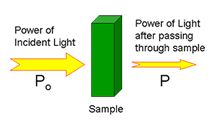
The technique is based on absorption of light. In molecular absorbance spectroscopy, a beam of ultraviolet or visible light is directed through a sample. Some of the light may be transmitted through the sample. Instrument measures transmitted light. Light that was not transmitted through the sample was absorbed. Absorbance (A) is defined as -log(P/Po).
Beer Lambert Law
States that absorbance of electromagnetic radiation by a given species is directly proportional to the concentration of the analyte. It is expressed as: A= εbC • where A is the absorbance, ε is the molar absorptivity, b is the path length and C is the concentration of analyte. Because ε and b are fixed under experimental conditions, the result is a linear relationship between absorbance and concentration.

Viscometer DV-II Brookfield
The Brookfield DV-II+Pro Viscometer measures fluid viscosity at given shear rates. Viscosity is a measure of a fluid’s resistance to flow. The Viscometer rotates a sensing element in a fluid and measures the torque necessary to overcome the viscous resistance to the induced movement. This is accomplished by driving the immersed element, which is called a spindle, through a beryllium copper spring. The degree to which the spring is wound, indicated by the red pointer, is proportional to the viscosity of the fluid.
All units of measurement are calculated in units of centipoise (cP); SI: 1 cP = 1 mPa•s
Loss on Drying (LOD)
A method commonly used for moisture content determination is the loss-on-drying method. Occasionally it may refer to the loss of any volatile matter from the sample.


Lyophilizer and freeze dryer are synonymous names for the same equipment. A lyophilizer executes a water removal process typically used to preserve perishable materials, to extend shelf life or make the material more convenient for transport. Lyophilizers work by freezing the material, then reducing the pressure and adding heat to allow the frozen water in the material to sublimate.
Controlled freeze-drying keeps the product temperature low enough during the process to avoid changes in the dried product appearance and characteristics. It is an excellent method for preserving a wide variety of heat-sensitive materials such as proteins, microbes, pharmaceuticals, tissues & plasma.
High-performance liquid
chromatography (HPLC)
High performance liquid chromatography or commonly known as HPLC is an analytical technique used to separate, identify or quantify each component in a mixture. HPLC works following the basic principle of thin layer chromatography or column chromatography, where it has a stationary phase and a mobile phase.
The mobile phase flows through the stationary phase and carries the components of the mixture with it. The mixture is separated and then identified and quantified by spectroscopy. A computer analyzes the data show the output in display.
